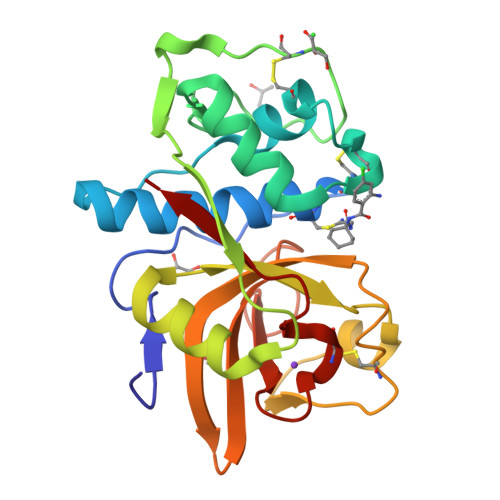
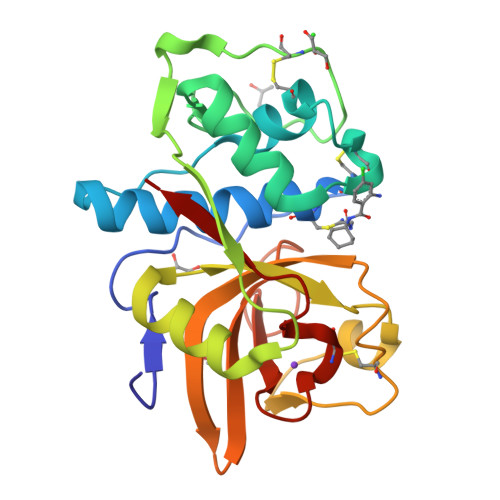
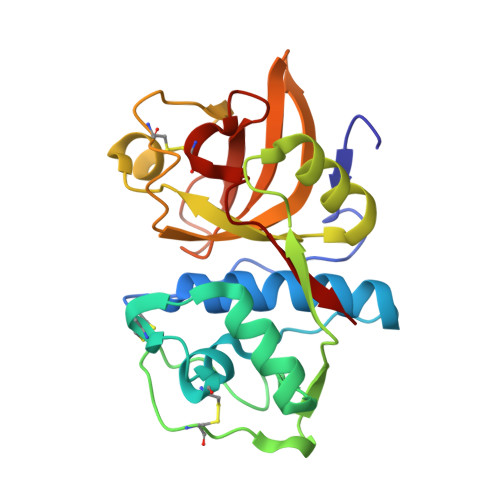
Development of N-(Functionalized benzoyl)-homocycloleucyl-glycinonitriles as Potent Cathepsin K Inhibitors.
Borisek, J., Vizovisek, M., Sosnowski, P., Turk, B., Turk, D., Mohar, B., Novic, M.(2015) J Med Chem 58: 6928-6937
- PubMed: 26280490
- DOI: https://doi.org/10.1021/acs.jmedchem.5b00746
- Primary Citation of Related Structures:
4X6H, 4X6I, 4X6J - PubMed Abstract:
Cathepsin K is a major drug target for osteoporosis and related-bone disorders. Using a combination of virtual combinatorial chemistry, QSAR modeling, and molecular docking studies, a series of cathepsin K inhibitors based on N-(functionalized benzoyl)-homocycloleucyl-glycinonitrile scaffold was developed. In order to avoid previous problems of cathepsin K inhibitors associated with lysosomotropism of compounds with basic character that resulted in off-target effects, a weakly- to nonbasic moiety was incorporated into the P3 position. Compounds 5, 6, and 9 were highly selective for cathepsin K when compared with cathepsins L and S, with the Ki values in the 10-30 nM range. The kinetic studies revealed that the new compounds exhibited reversible tight binding to cathepsin K, while the X-ray structural studies showed covalent and noncovalent binding between the nitrile group and the catalytic cysteine (Cys25) site.
Organizational Affiliation:
National Institute of Chemistry, Hajdrihova 19, SI-1001 Ljubljana, Slovenia.